You might be thinking about managing your autologous cell or gene therapy supply chain in-house. Or contracting with just one courier company for transport.
But our experience has proven that to overcome the challenges inherent to the cell therapy supply chain, you need just that. Experience. And you need multiple transport options to reduce your risk.
You need a skilled team coordinating, overseeing and optimizing every single step—from scheduling with all stakeholders to collection, manufacturing, and delivery to the patient. And you need multiple transport options to reduce your risk.
Our Autologous Cell Therapy Supply Chain Management service offers your cell and gene therapy company exactly that.
Our supply chain management model is unique
We are not a courier. We don’t rely on one courier or transport company. And we don’t oversee just one part of the supply chain. Instead, we support the full supply chain for every shipment of your autologous cell therapy.
Our Cell Therapy Supply Chain Managers are at the core of our solution. They see the whole picture of every cell therapy order.
Your Supply Chain Manager will:
- Coordinate with stakeholders across the supply chain from collection to manufacturing, and back to the patient
- Create patient- or therapy-specific delivery plans, including tracking and verifying chain of custody, chain of identity and certificate of compliance
- Schedule within parameters you set up, such as hitting manufacturing windows
- Communicate plans and itineraries with stakeholders
- Ensure stakeholders are kept up to date on any changes to plans or itineraries
This person serves as the single point of contact for your company and all stakeholders from start to finish. We have a Supply Chain Manager available 24/7/365 to ensure safe transport of your products if a transport issue occurs after regular business hours.
Let’s discuss how we can put that experience to work for you.
We’ve used the lessons learned over 35 years to build tools and processes that allow us to manage the variability inherent to cell and gene therapy supply chains.
Cell therapy managed logistics capabilities by the numbers
hours or less for continental U.S. delivery*
hours or less for international delivery*
annual cell and gene therapy shipments
of transports have an international component
*force majeure exempt
Mitigate supply chain disruptions with managed logistics
Managed logistics is how we coordinate the transport of your source material or manufactured therapy based on the needs of your product.
In our 35 years of experience managing the shipment of cellular therapies, we have found that it is important not to rely on a single courier. Different companies will have different strengths depending on the shipping lane and shipment type.
That’s why we have remained vendor agnostic.
Our team selects the provider that is the right fit for each product transport leg and provides the best itinerary possible. Sometimes this means using a combination of logistics providers to overcome a shipping challenge.
We know which commercial couriers are best for different shipping lanes. That allows us to balance risk and cost and optimize for transport success.
Our relationships with courier companies include:



Our Logistics Managers optimize your transport for success
Our Logistics Managers are behind the scenes of every shipment and are in close contact with your Cell Therapy Supply Chain Manager.
They schedule all legs of transport with the logistics providers that best fit your needs. And keep a close eye on your product to make sure it is safely delivered in the requested time frame.
Our team works in concert to provide reliable, regulatory-compliant transport and delivery. Every team member is working to make sure each cell therapy product is delivered:
- Where it needs to go
- With the proper documentation in place
- When it is expected to get there
- In the condition and temperature necessary
If needed, our Logistics Managers are able pivot quickly to meet your timeline because we use a managed logistics model.
“NMDP [BioTherapies] is the gold standard of logistics in cell-based therapies given the volumes that they manage.”
– Rob Jones
Vice President, Global Bioservices
Cryoport

Our established relationships worldwide allow for continued product delivery
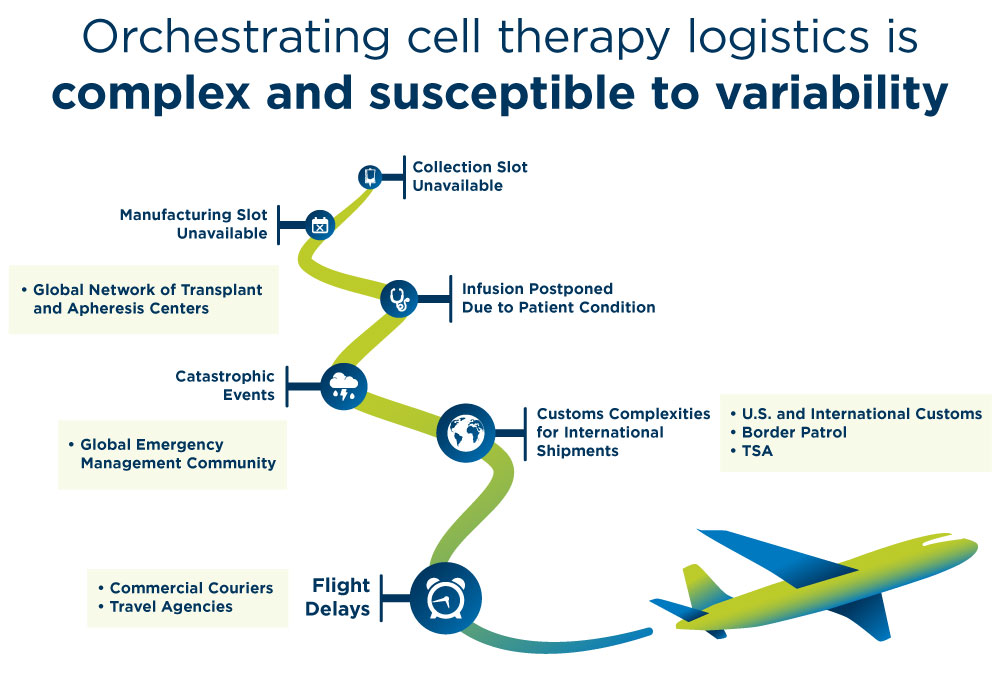
Despite well-designed cell therapy logistics plans, cell therapy supply chain disruptions are inevitable. And your risks increase exponentially as your therapy development operations scale.
Each new patient enrolled introduces multiple points for potential error or disruption in the delivery plan. Especially as you move from clinical trials to commercialization.
That’s why you need a partner with the infrastructure, processes and relationships in place to reroute, problem solve and deliver.
This is true for common issues, such as case delays, rescheduling or weather delays. It’s critical for major challenges, like the COVID-19 pandemic.
We always build contingencies into the supply chain for common issues. And our team knows how to mobilize in times of crisis to continue delivering time-critical products for patients.
We’re able to leverage established global relationships and troubleshoot supply chain disruptions via:
- Direct access to senior-level representatives at commercial courier companies
- Agreements with commercial courier companies for priority shipment loading
- Communication with global customs brokers for border crossing
Stay in the loop with our collaborative approach to troubleshooting
When unexpected disruptions happen, our Cell Therapy Supply Chain Managers know when to escalate issues to you. We work with all impacted stakeholders to problem-solve and optimize itineraries to ensure the new itinerary meets your needs.
Our team understands patients’ lives depend on meeting time-sensitive treatment schedules.
Every day, shipments go exactly as planned. But when issues arise, established relationships, collaboration and proactive communication are key to overcoming those challenges.
How can we help you?
There is no doubt the cell therapy supply chain is complex. But you don’t have to navigate the complexities of human-based therapy logistics alone.We’re ready to discuss your needs and how we can help.
Access additional services
Allogeneic Cell Sourcing
Lower your risk with end-to-end support for healthy donor identification, high-quality collections and cell delivery.
Collection Network Management
Receive ongoing management of your autologous cell collection network to maintain high-quality collections.
Consulting
Expertise to help you overcome domestic and international regulatory challenges.
Frequently asked questions
What delivery timeframes can NMDP BioTherapies comply with for my shipment?
We will work with you to develop a logistics plan that will fit your therapy’s needs. In general, we guarantee delivery in under 48 hours within the U.S. and under 72 hours internationally.
Is NMDP BioTherapies a courier company?
NMDP BioTherapies is not a courier company. Using a managed logistics model, we coordinate shipments with a variety of courier companies based on the shipping lane and your shipment type to optimize transport success.
What is managed logistics?
Managed logistics involves coordinating with a variety of courier companies, understanding their varying strengths, and adjusting utilization of these options based on the shipping lane and type of shipment. By taking this approach, we aim to optimize transport success.
Why would I contract with NMDP BioTherapies to manage logistics when I can contract directly with a courier company?
NMDP BioTherapies is an extension of NMDP℠, which has 30 years of experience managing the shipment of cellular therapies. This experience has shown us that it is important not to rely on a single courier. Each courier will have their strengths and weaknesses, depending on the shipping lane.
Because we manage a large volume of shipments, we are well suited to determine the optimal couriers for a particular shipment.
Does NMDP BioTherapies have preferred or recommended logistics partners? Can we ask you to use a specific partner or shipping container?
We will recommended logistics partners depending on the shipment type and location. We have flexibility to work with a variety of shipping partners and/or shipping containers. We will work with you to develop the optimal solution for your therapy’s needs.
What shipping containers does NMDP BioTherapies routinely use?
We have experience working with a wide variety of shipping materials. These will vary depending on the type of shipment. Some examples include:
- Cryoport
- DV10
- Credo Cubes
- Nanocoolers
How can I ensure my product is transported at the appropriate temperature?
All our shipments include a temperature monitor to track temperature through the life of the shipment. Additionally, we will work with you to ensure the optimal packaging is used to maintain the required temperature.
Are you able to transport material within the U.S. and internationally?
Yes, we have extensive experience transporting material across the globe.
Do you have experience transporting material at ambient/room temperature, cooled and cryopreserved temperatures?
We have a broad range of experience transporting material at a variety of temperatures. We can provide guidance on the packaging material and optimal shipping methods.
What is the difference between a Supply Chain Manager and Logistics Manager?
Our Cell Therapy Supply Chain Managers will manage and coordinate with all the appropriate stakeholders to create patient- or therapy-specific therapy delivery plans, including tracking and verifying chain of custody, chain of identity and certificate of compliance, and coordination with our Logistics Managers.
Our Logistics Managers manage our courier relationships and analyze shipping lanes to develop a transport plan. They monitor each product to make sure it is safely delivered in the requested time frame.
Let’s talk
Orchestrating cell therapy logistics and delivery is complex. We’re ready to discuss how we can help you mitigate your risk. And deliver on time.