When your cell and gene therapy company partners with NMDP BioTherapies℠, you gain access to a world-class, proven infrastructure that delivers high-quality allogeneic cellular source material.
Our standard GMP leukopak is designed to be delivered to you quickly without sacrificing quality. NMDP BioTherapies is the first and only company in the industry to include a drug master file (DMF) with our GMP leukopak. Optional add-ons to the standard leukopak—including cryopreservation—offer flexibility to meet your needs. This approach allows for ease in scale up as your therapy advances into later clinical trial and commercial phases.
Allogeneic cell sourcing through NMDP BioTherapies provides the reliability—and quality—your cell or gene therapy needs.
Are you developing an allogeneic cell therapy using cord blood as a starting material? We offer a central point of access to fully characterized cord blood units that simplifies acquiring the units you need. Access the details.
NMDP BioTherapies standard GMP leukopak
NMDP BioTherapies developed our clinical-grade leukopak offering with input from clinical- and commercial-phase cell and gene therapy companies. We’ve supported these companies for nearly a decade.
- Fresh, clinical-grade leukopak
- US. Food and Drug Administration (FDA) compliance
- Standard donor management
- Collections at qualified apheresis centers contracted to support standard leukopak collection
- Standard collection processes and documentation
- Standard logistics processes
- DMF on file
- Standard product cryopreservation
- Post-collection testing
- Health Canada (HC) compliance
- European Medicines Agency (EMA) compliance
- Therapeutic Goods Administration (TGA) compliance
- Specified donor demographics
- Additional donor samples for client-directed testing
- Additional donor testing prior to collection
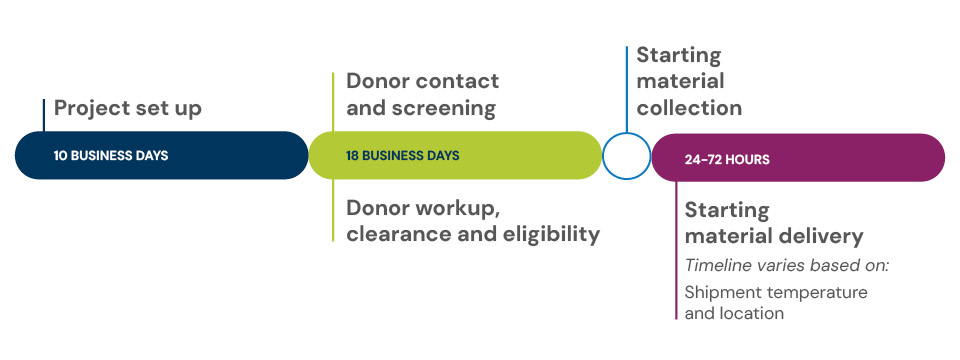
Typical timeline to first product delivery
Note: Manufacturing slot or other sponsor-required scheduling/receipt criteria may impact first product delivery.
When you select the standard fresh leukopak, you can expect to receive your first product delivery in six weeks. The typical timeline includes:
- Product set up in about 10 business days.
- Donor contact and screening plus donor workup, clearance and eligibility in about 18 business days.
- Starting material delivery of fresh products 24 to 72 hours after collection (timeline varies based on location and product being hand carried or shipped).
Varied timeline to first product delivery with add-ons
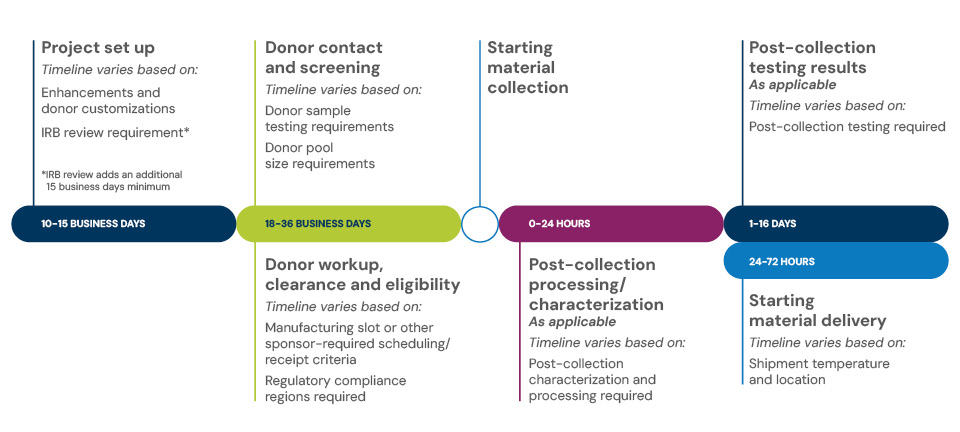
Selecting add-ons to the standard leukopak typically adds two to six weeks to the first product delivery. The timeline includes:
Product set up in 10 to 15 business days. Timeline varies based on:
- Enhancements and donor customizations
- IRB review requirements (additional 15 business days minimum)
Donor contact and screening plus donor workup, clearance and eligibility in 18 to 36 business days. Timeline varies based on:
- Donor sample testing and donor pool size requirements
- Manufacturing slot or other sponsor-required scheduling/receipt criteria
- Regulatory compliance regions required
Post-collection processing and characterization in 24 hours or less. Timeline varies based on:
- Characterization and processing requirements
Post-collection testing results in 1 to 16 days. Timeline varies based on:
- Post-collection testing required
Starting material delivery of fresh or cryopreserved products 24 to 72 hours after collection. Timeline varies based on:
- Shipment temperature and location
The standard GMP leukopak with or without add-ons is the most efficient option for cell delivery. NMDP BioTherapies offers additional made-to-order allogeneic cell sourcing services if you have more specialized requirements.
How can we help you?
We’re ready to discuss your allogeneic cell sourcing needs for your pre-clinical, clinical and/or commercial cell therapy development.
Stable source of GMP allogeneic donor cells
Cell and gene therapy manufacturers need a stable source of screened, healthy donors to decrease the potential for supply risk during scale up. Our volunteer donors are part of one of the largest, most diverse registries in the world: the NMDP RegistrySM.
HLA-typed potential
U.S.-based donors
new registry members
added in 2023
of new registry members in
2023 are ethnically diverse
We take extensive steps from the donor selection process through the donation process to deliver high-quality GMP leukopaks. Our donors most commonly donate:
- MNC-A
- Peripheral blood stem cells (PBSCs)
- Whole blood
Our donors are committed to advancing life-saving cell and gene therapies to help more patients. In fact, 94% of NMDP BioTherapies donors say they would go through the entire donation process again if asked.
High-quality leukopak collections for allogeneic cell therapy manufacture
NMDP BioTherapies contracts with 19 experienced apheresis collection centers throughout the United States. We aligned apheresis collection center locations with donor location density to reduce barriers to donation.
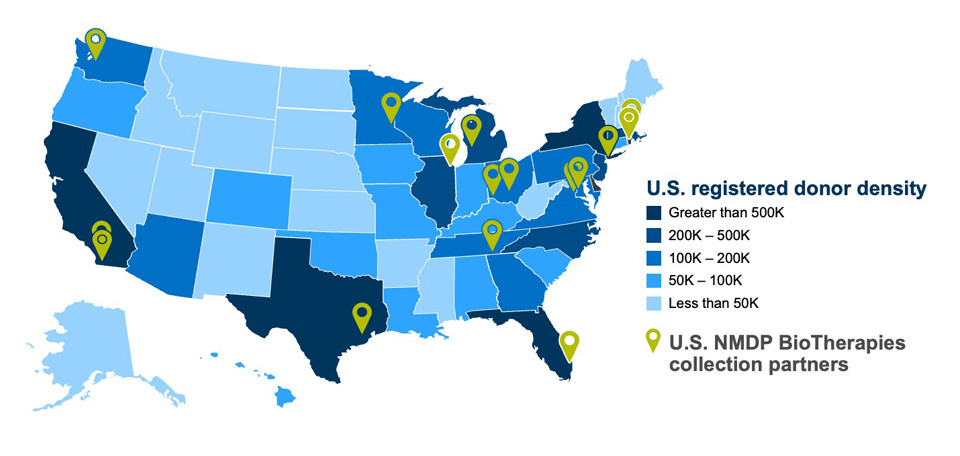
Apheresis collection centers in our network use a standard collection manual and batch record, which creates consistencies in processes and documentation. All collection facilities are FACT and/or AABB accredited and have completed a quality system audit.
Take the first step to receiving reliable, high-quality source material for allogeneic cell therapy development and commercialization. Contact our Sales team to discuss the standard leukopak and other NMDP BioTherapies products and services.
Access additional services
Cord Blood Unit Sourcing
Comprehensive visibility into the domestic CBU inventory and simplified sourcing.
CIBMTR® Bioinformatics Consulting
Expertise to help you optimize your allogeneic cell bank.
CIBMTR CRO Services
Insights and experience to successful lead your cell therapy clinical trial execution.
Frequently asked questions
What types of starting material can NMDP BioTherapies provide allogeneic cell therapy developers?
We most commonly supply MNC-A (leukopak), PBSC, whole blood and cord blood units. Contact us if there are other blood-derived materials you wish to collect for your therapy.
Do you compensate allogeneic donors?
We do not compensate allogeneic donors. We support voluntary, unpaid donations for clinically used cellular materials and products. This aligns with the policy of NMDPSM. We believe the use of non-compensated donors promotes donor and patient safety.
In addition, it aligns with international requirements and accreditation standards. Many international regions strongly emphasize or require the use of voluntary, unpaid donors for cell therapies. Non-compensation allows for the broadest use of a client’s allogeneic therapy.
What information can you provide about the donors on the NMDP Registry?
We have HLA information on all donors, as well as date of birth, self-identified race/ethnicity and gender. Additional characteristics, such as CMV status, may be available for subsets of the registry. Contact us to discuss your specific needs.
Why is it so important to have a donor pool that numbers in the millions? Which factors have the biggest effect on donor pool size?
Having access to a large donor pool is important to help ensure scalability in sourcing healthy donor starting material. This is true even for common donor characteristics.
The number of donor-specific attributes can have a significant impact on the needed donor pool size. The more donor attributes a therapy requires—such as viral positivity/negativity, specific HLA type, race or blood type—the larger the pool required.
As you add eligibility requirements, health authority and medical suitability requirements, study suitability requirements, and donor willingness to donate in the timeframe needed, your pool of potential donors will narrow.
Can you provide repeat donors?
If the donor is willing to participate, we can obtain repeat donations. Many of our donors are happy to provide repeat donations and are highly motivated by the potential to help save patients’ lives.
What is the advantage of using a contracted network of partners (i.e., apheresis centers, testing labs and clinics) instead of using internally owned centers?
NMDP has successfully used a contracted network model for more than 30 years for the facilitation of hematopoietic stem cell transplant. By using this distributed partner model, NMDP BioTherapies can support a large volume of allogeneic donors in diverse geographic regions. This also allows us to align your collection requirements with the centers that have the required capabilities.
Which source material regulations do you comply with?
Allogeneic donor source material regulations are country dependent. We comply with U.S. FDA regulations for cellular source material, including 21 CFR Part 11 (GMP) and 21 CFR Part 1271 (GTP).
We will collaborate with you to develop protocols that will meet international regulatory requirements that will support your intended markets. Our quality and regulatory teams have supported collections complying with Health Canada, EMA and TGA regulations.
Does NMDP BioTherapies consent allogeneic donors to use their cells for pre-clinical, clinical and/or commercial cell therapy development?
We work with each client to develop appropriate study-specific consent that will cover all intended uses, including pre-clinical research, clinical trials research and/or commercial use of the cellular source material.
Can you store cellular source material or the final allogeneic cell therapy?
We can discuss storage options with clients on a case-by-case basis.
Request a meeting
We know you have questions about us. We’d like to know more about your therapy, too. Let’s get the conversation started.