As a cell and gene therapy developer, your cell collection protocol requirements and specifications will impact your apheresis center network selection strategy and your ability to efficiently scale your processes. Any time your protocols or processes differ from those in place at a center, there are likely implications for training, forms and standard operating procedures (SOPs), which can delay your first collection.
That’s why an early, careful review of your collection process along the entire product collection pathway is so important. It is extremely valuable for cell and gene therapy developers to leverage the expertise of apheresis centers and use existing processes throughout the product collection pathway whenever possible.
Protocol decisions cell and gene therapy developers make along the product collection pathway can impact the ability to efficiently scale processes.
At Be The Match BioTherapies, we work with a large, established network of apheresis centers across the United States. Through our experience with these centers and cell and gene therapy developers, we’ve identified apheresis center best practices and standard processes that can help cell and gene therapy protocols get up and running as quickly and efficiently as possible.
Consider the processes and recommendations outlined below as you are developing and reviewing your own cell and gene therapy protocols. Making these decisions early will help you select the apheresis centers that are best suited to collect for your protocol.
Considerations for donor assessments and donor testing
Day of Collection Donor Assessments
Donor assessments done on collection day are an easy point for a cell and gene therapy developer to integrate the processes already used at apheresis centers.
Donor assessment standards at apheresis centers typically include:
- Identity verification using a minimum of two forms of ID, such as a government issued ID, verbal verification or medical record/institution verification
- Health History Screening Questionnaires (HHSQ) for healthy allogeneic donor assessments to verify donor suitability and eligibility for collection day donation
- Autologous collections can require study-specific assessments on collection day and additional time points as specified in your protocol
- Physical exam data point review, such as blood pressure, temperature or hemoglobin (these data points can vary depending on regulatory body requirements)
As you are developing donor assessment protocols, consider:
|
Day of Collection Donor Testing
When you are making decisions on donor testing timing, work towards minimizing the number of required blood draws on collection day. Each skin break increases infection risk.
We often work with clients to identify pre-collection donor testing requirements and the most efficient way to obtain those samples. For example, IDM testing may be required on collection day to meet certain regulatory body requirements. Other samples could be collected at a screening before collection day.
As you are developing donor testing protocols, consider:
|
Considerations for collecting starting material
Apheresis Equipment
The Spectra Optia is used most often by apheresis centers in our Be The Match BioTherapies Collection Network. However, some centers use other apheresis devices, like the Amicus.
Each center must validate the apheresis instruments implemented at the center and the SOPs in place for all protocols to deliver quality starting material. Any sponsor request to deviate from the center’s validated instruments or procedures can cause a delay because the center may be required to validate any collection protocol or procedure changes.
As you are developing apheresis equipment protocols, consider:
|
Processed Blood Volume
Apheresis professionals use processed total blood volume (TBV) as a key parameter for apheresis collections. They determine processing volume targets based on the donor’s size, sponsor’s requested cell count and product volume targets.
Be The Match BioTherapies assesses the product targets to determine what the likely processed blood volume would need to be in order to meet those targets. Our baseline standard is to process three times the donor’s total blood volume or 12 liters (whichever is less).
As you are developing processed blood volume protocols, consider:
|
Anticoagulants
Apheresis centers use anticoagulants to prevent product clumping during collections. An apheresis center will have SOPs in place for anticoagulant use unless the sponsor restricts the use of specific anticoagulants.
For example, if heparin has negatively impacted manufacturing results, the sponsor may require that centers use a specific anticoagulant, like ACD-A.
As you are developing anticoagulant protocols, consider:
|
Concurrent Autologous Plasma
Before collection is complete, apheresis centers can collect concurrent plasma from the donor and either add it to the final product or send it with the starting material in a separate bag. Adding additional concurrent plasma is particularly beneficial for fresh products that are being shipped long distances. The plasma has a buffering effect and helps in maintaining cell viability.
However, this practice is less common for products that are being cryopreserved onsite due to the plasma reduction step required for cryopreservation.
As you are developing concurrent autologous plasma protocols, consider:
|
Product Hematocrit and Volume Target
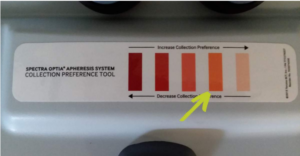
Example of a colorgram that apheresis operators use to reach product hematocrit targets.
Apheresis operators can work towards meeting specific material specifications, such as product hematocrits and volume targets.
Visual indicators, like colorgrams, allow the apheresis operator to reach specified product hematocrit targets. These targets range from 1 to 3% based on the sponsor’s specifications.
Product volume targets allow the apheresis operators to gauge processing volumes. Your volume target has implications on:
- Materials used during collections, such as the size of the leukopak
- Materials used for processing and shipment, such as the size of the cryobag
- Downstream processing steps
As you are developing product hematocrit and volume target protocols, consider:
|
Product Labeling
Example of a leukopak base label used on cell therapy products.
ISBT-128 is the global labeling standard for blood, cell and tissue products. An apheresis center must implement ISBT-128 labeling to receive FACT or AABB accreditation.
If your products require additional labeling, it will be important for the apheresis center to have the label prior to the scheduled apheresis dates.
Your label size will have implications on how all labels are positioned on the leukopak base label.
As you are developing product labeling protocols, consider:
|
Considerations for post-collection processing
Product Processing
Apheresis centers use validated SOPs for any processing services they offer, such as cryopreservation. Sponsor-required deviations from those validated procedures may require revisions to the center’s SOPs along with validation of the new procedure prior to use. In addition, centers will require training on your protocol-specific steps.
These steps can all impact your collection timeline.
For some protocols, deviations from a center’s SOPs and procedures will absolutely be needed. However, if you have no processing restrictions, leveraging the center’s expertise and procedures is the most efficient path forward.
As you are developing product processing protocols, consider:
|
Post-Processing Requirements
After collection and processing, the center will need to know how you want the product volume to be partitioned or aliquoted for the manufacturer. Additionally, the center will need to know if the manufacturer has limitations on when they can receive starting material.
While these appear simple, they have implications much earlier in the process.
How the product is partitioned can impact the materials selected for shipment, such as the size of the cryobags used for cryopreservation. If a manufacturer only accepts shipments on specific days or times, it will impact when the donor is scheduled for collection.
As you are developing post-processing requirements, consider:
|
Apheresis center capabilities assessment
Once you have developed your scalable collection protocol requirements and specifications, you can begin assessing an apheresis center’s capabilities along the product collection pathway. Not all apheresis centers will have the specific capabilities or skillsets to collect for your protocol.
When assessing an apheresis center’s capabilities, ask questions like:
- Is the center capable of delivering on your required specifications?
- Does the center have enough collection slot availability to meet your timeframes and volumes?
- Can the starting material get from the apheresis center to the manufacturing center in the required delivery timeframe?
Organizations like Be The Match BioTherapies that have established collection networks are ideally suited to assist you with this capabilities assessment. This approach offers many advantages for cell and gene therapy sponsor including:
- The organization understands the strengths and weaknesses of each center and can target the centers most likely to be able to deliver on your requirements.
- The sponsor benefits from efficiency across the supply chain when the organization has an integrated collection network and managed logistics team.
- The organization can leverage its experience to help you implement best practices and processes in your requirements to efficiently get your cell therapy protocols up and running.
Remember, many crucial considerations will impact your center selection strategy and your ability to efficiently scale your processes. Think through your process early to maximize quality and scalability throughout your supply chain and leverage existing expertise for efficient protocol implementation.
Collection Network Onboarding for Cell and Gene Therapies
Be The Match BioTherapies prepares apheresis centers to collect source material for a cell and gene therapy sponsor’s clinical trial or commercial therapy.





