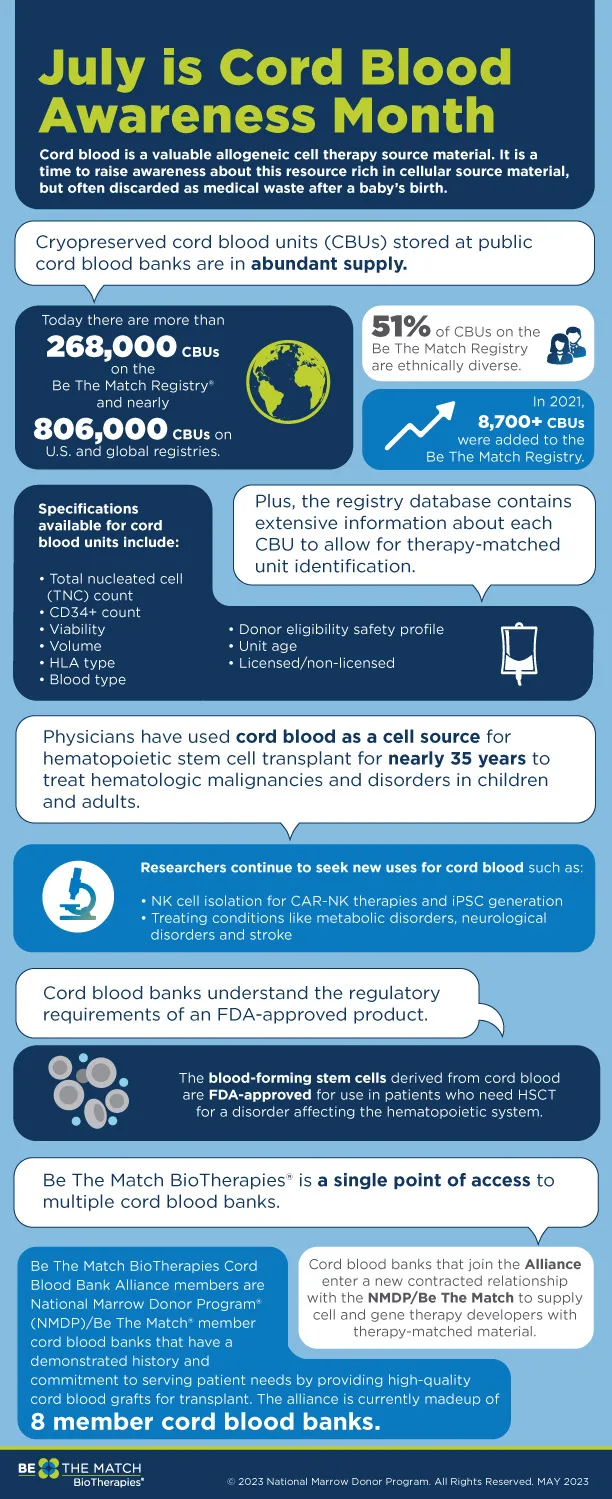
The newly formed NMDP BioTherapies℠ Cord Blood Bank Alliance (CBBA) has the potential to open the door to new cellular therapies by expanding awareness and enabling researchers and developers to more easily identify and access cord blood units (CBUs) for their work.
Cord blood is an abundantly available biologic resource with a proven safety profile that can be leveraged to develop critical, potentially life-saving cellular therapies. What was once discarded as medical waste, cord blood is now recognized as a valuable source of cellular starting material that is simple to collect and store, rapidly available and is less likely to promote an immune response.
While cord blood has been used in hematopoietic stem cell transplant for decades, its full potential has not yet been tapped. Cell and gene therapy researchers have demonstrated the unique value of CBUs as a cellular starting material. For example, researchers have isolated NK cells from cord blood for CAR-NK therapies as well as using cord blood for induced pluripotent stem cell generation.
The opportunities presented by the NMDP BioTherapies CBBA will help advance research into new cellular therapies, using this important but often over-looked resource.
The NMDP BioTherapies CBBA brings together leading cord blood banks with decades of experience managing the development, production and distribution of allogeneic products in a regulated environment. The NMDP BioTherapies CBBA currently includes eight NMDP℠ member banks that have demonstrated commitment to serving the needs of patients and providing high quality cord blood units for the development and commercialization of emerging cell therapies. They include:
- Bloodworks Northwest
- Cleveland Cord Blood Center
- Carolinas Cord Blood Bank
- Cord for Life
- StemCyte
- South Texas Blood & Tissue
- Versiti
- Vitalant
NMDP BioTherapies will act as the single point of contact for contracting and quality system engagement between researchers and developers seeking therapy matched CBUs, and cord blood banks. It will provide visibility into the NMDP Registry℠ of 270,000 clinical grade, fully characterized CBUs in domestic banks, and manage the logistics to ensure CBUs are delivered to developers when needed.
The United States government selected NMDP operate as the nation’s Cord Blood Coordinating Center as mandated by the Stem Cell Therapeutic and Research Act of 2005 (Stem Cell Act 2005) and amended by the Stem Cell Therapeutic Reauthorization Act of 2010 and 2015. In this role, the organization works with public cord blood banks, doctors and researchers to continually improve cord blood transplantation and educate medical professionals and the public. This work expanded the organization’s deep relationships with cord blood banks and positioned it to take a leadership role in creating the CBBA.
Explore the many ways the NMDP BioTherapies can support your therapy development when cord blood is your source material: Cord Blood Units for Allogeneic Cell Therapy Source Material