The rapid growth of the cell and gene therapy industry is a positive for patients. However, with that growth comes the increasing demand for high-quality cellular source material collected at apheresis centers.
The pace of cell and gene therapy development
As of early January 2022, the FDA has approved five CAR-T treatments for hematologic malignancies as well as two gene therapies to treat rare diseases. The pipeline for cell and gene therapy products remains strong.
According to the Alliance for Regenerative Medicine’s (ARM) H1 2021 report, more than 2,600 regenerative medicine and advanced therapy clinical trials are underway globally. These include industry-sponsored trials as well as those sponsored by non-industry groups, including academic centers and government. More than 240 of those trials are in Phase III.
The ARM H1 2021 report also shows a rapid increase in the development of unique products in the industry-sponsored sector alone. Their report showed that there were 956 unique therapies in development through the first six months of 2021. That number is forecasted to grow to more than 3,100 by 2026.
Forecast indicates a three-fold increase in apheresis collection demand
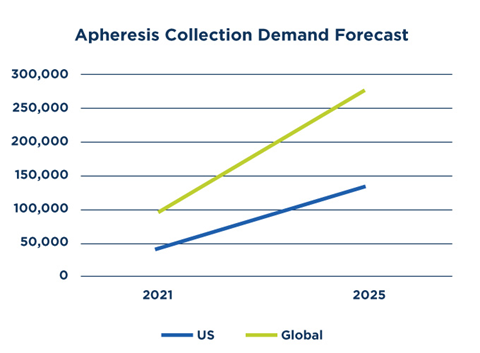
Forecast data from Accenture demonstrates just how quickly the need for apheresis collections may grow in the United States and globally as the number of cell and gene therapy products in development grows. (Countries included in global forecast data are the U.S., China, Japan, Germany, Australia, U.K., South Korea, France, Italy and Spain.)
The forecast includes anticipated apheresis patient collection volume for:
- Approved therapies
- Clinical trials
- Future approved therapies
In the U.S. alone, collection demand is expected to increase from approximately 43,000 annual collections in 2021 to 132,000 by 2025—a three-fold increase.
Managing the collection capacity impacts from this growth will require understanding challenges from the perspective of the apheresis center and collaboration across the industry to develop solutions.
Miscommunication is common and can lead to inefficiency
Apheresis centers and cell and gene therapy sponsors may use the same words, but they are not always speaking the same language.
“We get these inquiries within our research division that just say, ‘I need a leukopak.’ But what does that mean? Do you care about volume? Do you care about TNC? Do you care about MNC? Do you care about CD3? There have been times we’ve learned that sometimes they just assume and you have to ask,” said Stephanie Dormesy, MPH, Director of Cellular Therapy Donor Management at New York Blood Center.


Lacey Anderson is the Senior Manager of Collection Network Management for Be The Match BioTherapies®. She recalled a conversation with a cell and gene therapy sponsor that—without a clarifying question—could have had major implications in a center’s ability to collect for the protocol.
“It sticks out in my mind vividly. The sponsor said, ‘We want GMP processing.’ And I remember asking, ‘What do you mean when you say that? This is what it means to me. What does it mean to you?’ They wanted a clean room. We were not talking about the same thing,” Lacey said.
A questionnaire can identify needs and ensure a center has the needed capabilities to collect for a protocol
Some centers have created processes to alleviate confusion from the very beginning.
“We’ve created a questionnaire we provide new clients. It’s very detailed so we can pinpoint exactly what’s needed. We’ve been working to standardize our flow so each customer’s requirements—whether they have minimal requirements or maximum—are very clear cut so we know what exactly they need,” Stephanie shared.
It’s a step Be The Match BioTherapies takes with its clients, too.
“We are trying to bridge that language barrier to make sure we are all on the same page from the beginning. Our goal is to reduce confusion right away. We can start moving on the same path earlier and increase efficiency and reduce complications and incidents down the road,” Lacey explained.
Standardization in protocols (when possible) can help increase capacity
Currently there is huge variability from protocol to protocol and from center to center. If cell and gene therapy sponsors and apheresis centers work together to standardize and streamline where possible, it can help increase capacity in the future.
“The variation from protocol to protocol can range so drastically. That range can have a great impact on the time it takes to collect and process at an apheresis center,” Lacey explained.
Be The Match BioTherapies works with sponsors from the beginning to figure out what is a must have and what is a nice-to-have. Post-collection processing is one example.
“Post-collection processing especially can be very time consuming. We’ll ask clients what the apheresis center really needs to complete and what could be done later. Getting the product collected and out the door with minimal processing at the apheresis center could hopefully free up some capacity,” Lacey said.
Sponsors can create efficiencies by allowing centers to follow their existing standard operating procedures (SOPs)
While some post-collection protocol variations are absolutely necessary, others may not be. In these cases, cell and gene therapy sponsors and apheresis center staff alike can benefit from using the center’s existing SOPs and procedures.
“When we are working with Be The Match BioTherapies clients, this is an area where we really try to help sponsors understand what they really need. It’s about taking the time to bridge the gap to figure out the reason behind the request,” Lacey explained.
She added, “We’ll ask them a lot of questions about why they’re requesting aliquots, for example. Is it necessary? Does it impact the manufacturing process? If not, is it necessary to deviate from a center’s standard processes? We’re trying to help everyone find the most efficient way forward.”
The apheresis collection itself is one of the key areas for cell and gene therapy sponsors to consider standardization.
“We try to be an advocate for the center and ask that the sponsors let the centers collect the way they collect. Let’s not disrupt their process, especially the collection process. Let’s let them follow their SOPs,” Lacey said.
“A lot of times sponsors want to come in and tweak [the collection process]. But the procedure is the procedure. Keep it standardized,” added Hope Guidry-Groves, Senior Director of Network Partnerships at the National Marrow Donor Program® (NMDP)/Be The Match® and former Director of Cellular Life Solutions at Gulf Coast Regional Blood Center.

The lack of standardization in documentation and portals is inefficient for staff
Variation in documentation and the documentation entry portals create another challenge for apheresis centers.
“As we expand, there are multiple portals the nurse has to go into to document for apheresis. It’s not just one different portal. It’s two. It’s three. It’s four. Each one is just a little different and how exactly you define each data element may be a little bit different for each company,” said Heather Steinmetz, MPH, Quality Assurance Manager for the Hematologic Malignancy and Stem Cell Transplantation program at UCLA Health.
She suggested a standard gathering portal that each company would use.
“You say, ‘Ok, I’m collecting this product, here’s the company’s questions, transmit to that company.’ Just one login point so that visually it looks the same for the nurses because this is over and above their normal documentation. Some standardization and ease of entry would be helpful,” she added.
How apheresis centers are preparing for the future
Many apheresis centers are taking steps now to prepare for the influx of collection requests in the future.
For example, New York Blood Center has frequent meetings to discuss staffing and equipment to ensure they are ready for future growth.
“We all know the cell and gene therapy industry is growing exponentially. We don’t want to race [to add staff and equipment] at the same time that we’re getting these requests. Rather than waiting for the overflow, we’re game planning to see what areas we can grow,” Stephanie shared.
UCLA Health changed its staffing patterns to increase capacity.
“We could not increase our space. So, we’ve chosen to increase the amount of time we use the space and equipment we have,” Heather explained.
They did so by transitioning most of their nursing staff to 12-hour shifts instead of eight- or 10-hour shifts. “Now we have not just the morning and afternoon shift, but a morning, a midday and an evening shift,” she said.
As they look to the future, Stephanie, Heather, Hope and Lacey all agree that collaboration across the industry is key and conversations must continue.
Lacey summed it up saying, “The more people we can get working on all the aspects of a collection—from the collection itself to processing, packaging, shipping and even training—the better off the industry will be and the further we’ll go faster.”
Get streamlined apheresis center onboarding and training for your autologous collection network
You need consistent, high-quality source material for your autologous cell and gene therapy study or commercial therapy. Our team can help. After assessing a center’s infrastructure and capabilities to collect for your protocol, we train the center to collect for your therapy.
Learn how we set your apheresis center network up for success:
Collection Network Onboarding for Autologous Cell & Gene Therapies