Sourcing your starting material from high-quality, healthy donors matters when you are manufacturing an allogeneic cell therapy. Any misstep can mean delays in your allogeneic therapy’s development. That makes your choice of a cell sourcing vendor critically important.
Our team has sourced and collected cells for time critical cell therapies from more than 100,000 volunteer allogeneic donors. We’re providing the insights we’ve gained over nearly 35 years to you.
Use these six considerations when you’re evaluating vendors to identify donors, collect source material and deliver it for manufacture.
1. Donor pool depth
You may not need a high volume of donors when your allogeneic cell therapy is in an earlier clinical development stage. However, your donor pool will need to grow as your therapy scales into later stage clinical trials and gains commercial approval.
By working with a partner from the start that has a large donor pool, you can quickly scale your volume. Be The Match Registry® is an example of a large donor pool. The more than 22 million potential adult donors on the registry are HLA typed and represent diverse segments of the population.
A large donor pool like this is particularly important when your therapy requires specific or rare donor attributes. With each attribute added, your donor pool will shrink. (Figure 1)
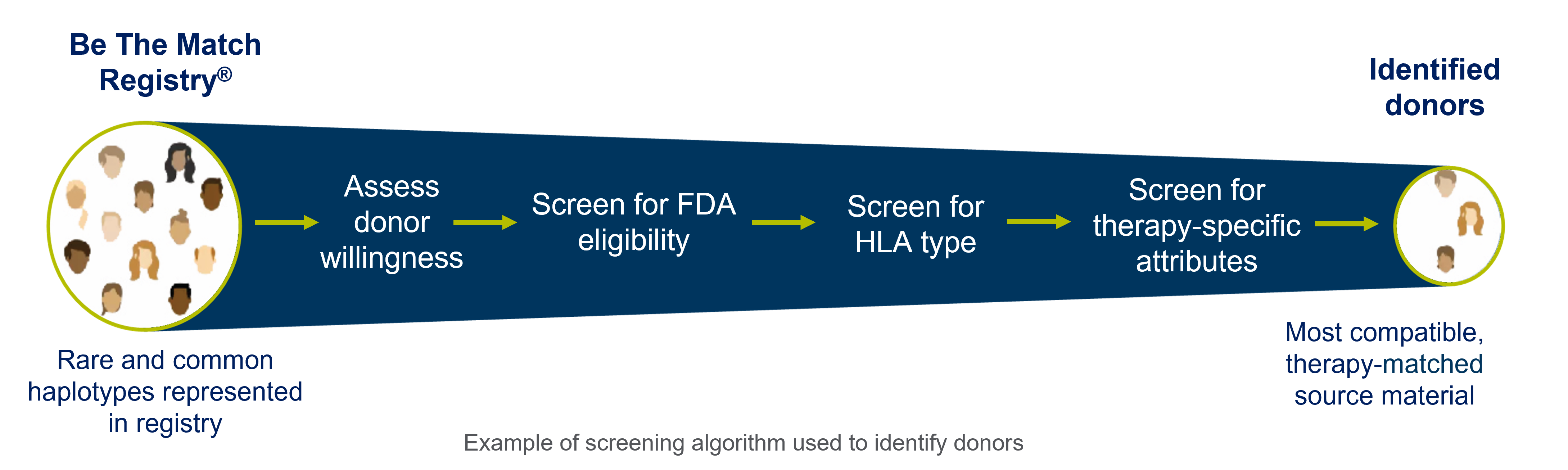 Partnering with an organization with a large donor base makes it more likely a donor with your specific attributes can be sourced and collected in your desired time frame.
Partnering with an organization with a large donor base makes it more likely a donor with your specific attributes can be sourced and collected in your desired time frame.
When evaluating a company’s donor pool depth, ask:
- What is the size of your donor pool?
- Are your donors geographically distributed or local to one city or region?
- Do you have HLA type information available? To what resolution?
- Do you have race and ethnicity information about your donors?
- Do you have, or can you obtain, a donor’s serostatus for infectious diseases, such as CMV or EBV?
- How often does your company rely on repeat donors? What is the average number of times you are able to get a donor to repeat donations?
2. Scientific expertise
An experienced bioinformatics team can help your company:
- Determine the attributes that are the most important for your therapy
- Establish the size of the donor pool you’ll need
- Identify compatible donors that meet your criteria
For example, our Bioinformatics team assists clients with algorithm development to both source donors and get the right therapy to the right patient. The team has more than 30 years of experience conducting sophisticated bioinformatics.
When considering a company’s scientific expertise, ask:
- Do you have experience evaluating potential donor matching requirements to increase therapeutic success?
- Can you help determine the population frequencies for the donor attributes needed for my therapy?
- Can you help in matching the ideal manufactured cell bank to each patient?
- Is your team up to date on matching science and practices?
3. Donor pool quality
A quality donor is one who accurately answers the health history questionnaire and shows up for exams and collection on the agreed upon dates.
If donors do not accurately portray their health history, the source material might not be usable if issues are detected after donation. Donors who don’t show up for collection can cost you your manufacturing slot. This can delay the manufacture of your therapy and your path to commercialization.
When donating for altruistic reasons, our experience shows donors have a higher likelihood of:
- Accurate health history reporting
- Attending scheduled appointments
- Showing up for collection
For example, through the National Marrow Donor Program®/Be The Match®, we’ve facilitated more than 100,000 cell collections for time-critical cellular therapies. Our volunteer allogeneic donors are not compensated for their donated product or time. They receive an extensive health history screening and education. No-shows on collection day are extremely rare.
Ask potential cell sourcing partners:
- What is your donor no-show rate (i.e., not showing up for appointments or collection)?
- Do you compensate your donors in any way (i.e., for their donated product or their time)?
- Do your company policies meet EU regulations for compensation of donors?
Finding this information on selecting a cell sourcing vendor useful? Download it as a PDF.
4. Donor screening process
Collecting quality source material requires an extensive donor consent, screening and testing process. This process is necessary to make sure each donor is willing, safe and suitable to donate for your specific therapy.
A robust donor consent, screening and testing process starts with the initiation of donor contact. It spans through infectious disease marker (IDM) testing post-donation. The process should meet FDA and international requirements for research, clinical and commercial uses.
This is an example of the multi-step process our Be The Match BioTherapies® team uses for each donor. (Figure 2)
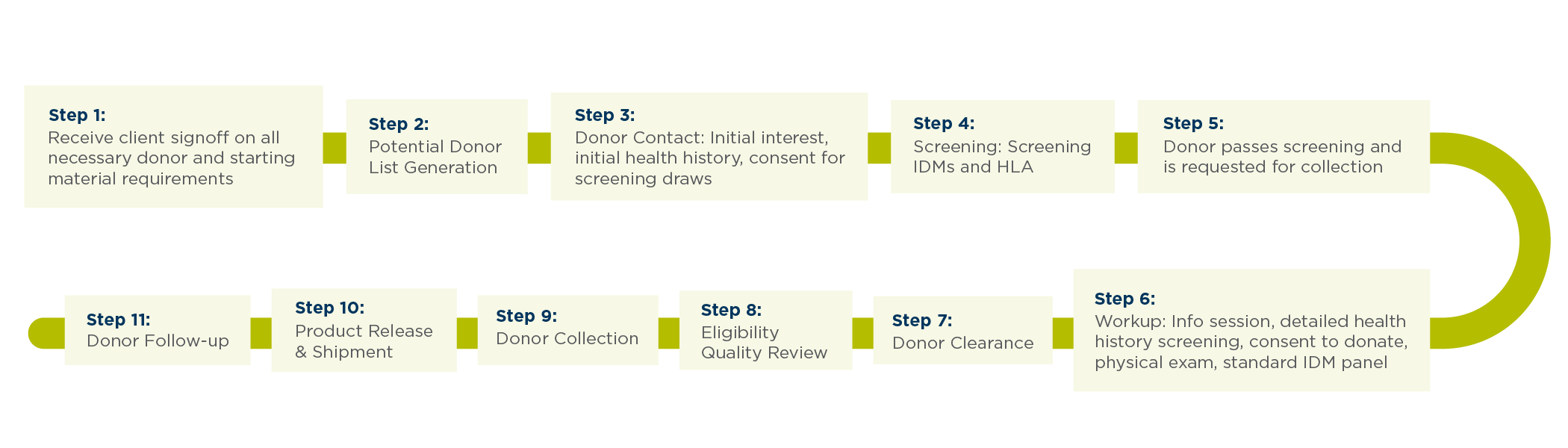 When selecting a partner to source healthy donors for starting material collection, ask:
When selecting a partner to source healthy donors for starting material collection, ask:
- How will you identify and contact donors regarding this opportunity to donate?
- What is your process for consenting donors?
- Can you perform testing for infectious diseases outside of regulatory requirements for tissue (e.g., HHV 6/7/8, adenovirus, etc.)?
- Can we provide additional therapy-specific screening criteria outside of the normal screening criteria your company uses?
- How do you educate donors about therapy-specific donations?
5. Cell collection network depth
You need to know the centers you select to collect source material for your therapy can deliver high-purity product. Not every apheresis or marrow collection center is the same. Qualifying, auditing and onboarding each separately takes significant time and resources.
Partnering with an organization with an extensive cell collection network that has implemented best practices and standardized protocols can help ensure source material consistency.
For example, we built our U.S. Collection Network of 84 apheresis centers and 67 marrow collection centers over 30 years. We’ve developed partnerships in the EU to support clients developing therapies internationally.
That allows our team to partner with clients developing cell therapies to fully understand their individual therapy and regulatory compliance requirements. We determine the best-suited apheresis or collection centers for the therapy. To support more consistent and high-quality collections and therapy infusions, we provide extensive onboarding, training, and ongoing support and management.
When selecting a partner to collect your source material, ask:
- How many collection facilities do you have in your network?
- Are your collection facilities geographically distributed across the country or central to one region?
- How do you train your collection facilities to meet the requirements of our protocol?
- How do you ensure your collection facilities collect high-quality products?
- Are your collection centers FACT and/or AABB-accredited?
- Has your organization collected source material to support U.S. and European-based cell therapy developers?
6. Logistics experience
Speed of delivery after collection is critical to cell viability. Cellular source material must be delivered:
- Where it needs to go
- When it is expected to get there
- In the required condition and temperature
When that source material is traveling hundreds or thousands of miles to a manufacturing facility, unexpected disruptions can occur. Working with a partner that is skilled at overcoming these challenges is a critical part of your therapy’s success. Established relationships and contingency plans are key to overcoming disruptions.
Our more than 60-person Cell Therapy Supply Chain Management team has seen the importance experience brings. Our Cell Therapy Supply Chain Managers and Logistics Managers have been coordinating logistics for a time-sensitive cell therapy, hematopoietic stem cell transplant, for decades. They successfully deliver product within the continental United States in 24 hours or less, and internationally, barring force majeure, in 48 hours or less.
The team has learned to successfully overcome common challenges, such as weather delays. They also have the relationships in place to mitigate less common challenges, such as customs complexities or disruptive world events like the COVID-19 pandemic.
When selecting a logistics partner, ask:
- How many years of experience does your organization have providing logistics to cell and gene therapy developers?
- How large is the team that will support the logistics activities for our product? Who are the team members?
- How many cellular therapy product shipments a year do you manage?
- How often do your shipments cross international borders? What processes do you have in place to overcome border-crossing issues for cell-based products?
- Does your company provide after-hours, weekend and holiday on-call support to manage unexpected changes and emergencies?
- What is your company’s experience managing the transport of fresh products? Cryopreserved products?
- What experience does your company have providing a high-touch service, such as a hand-carried courier service?
Related content
5 Qualities to Seek in Your Cell Therapy Supply Chain Vendor (blog)
Access allogeneic source material you can count on.
Explore our Cell Sourcing Solutions. Then connect with us to discover how we can help you minimize your risk and deliver high-quality source material to you the first time.





