Cells from adult donors are not the only source material allogeneic cell and gene therapy developers should consider. Cord blood is a valuable starting material, too.

Marcie Finney is the executive director of the Cleveland Cord Blood Center. The center is a founding member of the Be The Match BioTherapies® Cord Blood Bank Alliance. The Alliance provides a single point of access to the domestic cord blood inventory and centralizes cord blood bank qualification and contracting.
Cord blood’s unique, naïve biology often comes up as a main reason to use it. But there are four non-biological factors cell and gene therapy developers should also consider.
1. Cord blood bank staff are ingrained with a GMP mindset
Cord blood is a highly regulated product. Eight U.S. cord blood banks have FDA-licensed products for use in hematopoietic stem cell transplant (HSCT).
That means the FDA has approved a bank’s process for making umbilical cord blood units (CBUs) for HSCT. This offers an advantage for cell and gene therapy developers even though the indication is different.
I’ll use our cord blood bank—the Cleveland Cord Blood Center—as an example. The FDA approved our biologics license application (BLA) in 2016.
Everything we do is modeled under that BLA mentality.
The way we train our people and how they work. The way our facilities are designed, maintained and monitored. The way our equipment is validated and monitored.
That GMP mindset is ingrained.
Why is that important for cell and gene therapy developers? It’s a big jump to move from the research lab to a GMP-like facility for clinical trials and commercial products. Everything that touches that product, every person involved in its manufacture, and every place that product moves through must be thought about in a GMP manner.
Our staff already know how to manufacture the CBUs in a way that the FDA approved.
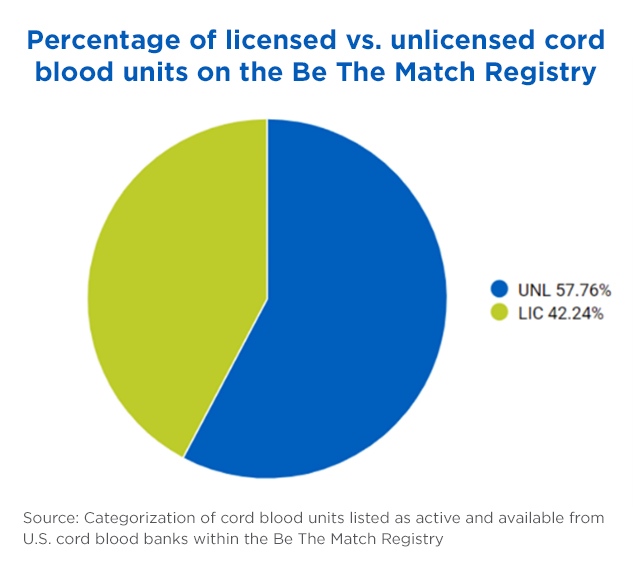
Nearly 58% of cord blood units available on the Be The Match Registry are unlicensed, while 42% are licensed.
2. Unlicensed does not mean inferior when it comes to cord blood units (and increases the available supply)
Confusion over licensed versus unlicensed CBUs exists. Many people think unlicensed CBUs are inferior to those that are licensed.
Licensure really means that a cord blood bank’s operations have been reviewed and accepted by the FDA for the way these products are made.
It doesn’t necessarily mean the biological properties of the products are superior.
Both licensed and unlicensed units meet donor eligibility and safety requirements for use in humans, and physicians use both clinically in HSCT.
There are, in fact, more unlicensed domestic units available on the Be The Match Registry® than licensed units.
If your cell therapy company doesn’t focus exclusively on licensed units, you’ll have a better likelihood of having a higher volume of units available for use in your therapy.
Licensed and unlicensed units have two main differences.
One is simply timing.
Any CBUs processed and stored before a bank received approval of its BLA cannot be licensed. Remember, licensure is specific to the BLA for HSCT.
In our case, we started our cord blood bank in 2008. We received our FDA license in 2016. Even though our processes and equipment had not changed, the CBUs we manufactured before 2016 cannot be licensed.
The second difference is that unlicensed CBUs are missing one or more specifications required in the BLA to support allogeneic transplant. For example, a donor may have traveled to a Zika risk area or a unit may be missing a sample specified in the bank’s BLA.
The unit could still be extremely valuable for a cell therapy product.
3. Cord blood units are readily available and highly characterized
Cell and gene therapy developers can encounter donor availability or eligibility issues when they use adult donors for source material. Those are not issues they will experience with cryopreserved CBUs.
Within 48 hours of collection, banks process and test the units. While the process for creating the units may differ slightly from bank to bank, cord blood banks upload standard information to the same searchable database.
This includes information on viral testing, cell viability characteristics such as total nucleated cell (TNC) counts and CD34+ cell counts, HLA type and more.

Cord blood is truly an off-the-shelf product. With safety, purity and potency testing already complete, the product can get out the door quickly.
Current available data points are specific to those needed for HSCT. However, with consensus from the cell and gene therapy industry, cord blood banks could likely increase the product characterization tests to include data important to cell and gene therapy developers.
4. An Alliance and an established database offer a single point of access to the cord blood inventory
Cell and gene therapy developers can obtain a single point of access to the domestic cord blood inventory through the Be The Match BioTherapies Cord Blood Bank Alliance.

Be The Match BioTherapies centralizes cord blood bank qualification and contracting. Cell and gene therapy developers work through one entity—Be The Match BioTherapies—to access CBUs.
This creates needed efficiencies for both cell and gene therapy developers and cord blood bank staff. Cell and gene therapy developers do not spend time and effort qualifying and contracting with each individual bank and vice versa.
The Cord Blood Bank Alliance also gives cell and gene therapy developers a central look at the cord blood inventory in an established database developed and managed by the National Marrow Donor Program® (NMDP)/Be The Match® (Be The Match BioTherapies is part of the NMDP/Be The Match).
It’s like how transplant centers access the cord blood inventory today.
Transplant centers do not contract with individual banks. They don’t contact us directly for the CBUs they need.
Instead, they have a single point of access for searching and ordering cord blood units—the NMDP/Be The Match.
Explore cord blood as a cell and gene therapy source material
Cord blood bank regulatory experience, the off-the-shelf nature of cord blood and the ease of obtaining cord blood units only scratch the surface of the benefits cord blood holds as a source material for cell and gene therapies.
Be The Match BioTherapies and the Cord Blood Bank Alliance can help you understand all the benefits cord blood has to offer for allogeneic cell therapy development:





