Expertise and experience to successfully lead cell therapy clinical trial execution.
CIBMTR® CRO Services for Cell and Gene Therapy
Expertise and experience to successfully lead cell therapy clinical trial execution.

A history of advancing cell therapies
Translate complex cell therapy study designs into operational excellence

Benefit from our unique infrastructure and extensive clinical trial site network
Because the CIBMTR is a research affiliation between NMDP℠ and the Medical College of Wisconsin, our partners can leverage a powerhouse of support and the established infrastructure and stability that goes along with it.
We are both a sponsor running our own clinical trials and a CRO running trials for other organizations. Our research organization is embedded within the operations of NMDP. That means our partners benefit from the synergies with NMDP and NMDP BioTherapies℠ for:

End-to-end clinical trial design, operations and logistics support

Built-out clinical infrastructure with single Institutional Review Board (sIRB), dedicated Data Safety Monitoring Board (DSMB), master contracts and 21 CFR part 11 compliant technology

Access to patients and allogeneic donors for research

A direct link to the CIBMTR Outcomes Database with information on more than 700,000 patients
Active investigational transplant center sites in our clinical trials network
We harness established relationships with a contracted network of nearly 250 investigational transplant center sites as well as donor center and research lab partners across the United States.
Our established relationships and contracts with investigational sites allow us to successfully recruit sites to participate in trials, complete site start up faster and expedite processes throughout the duration of the study.
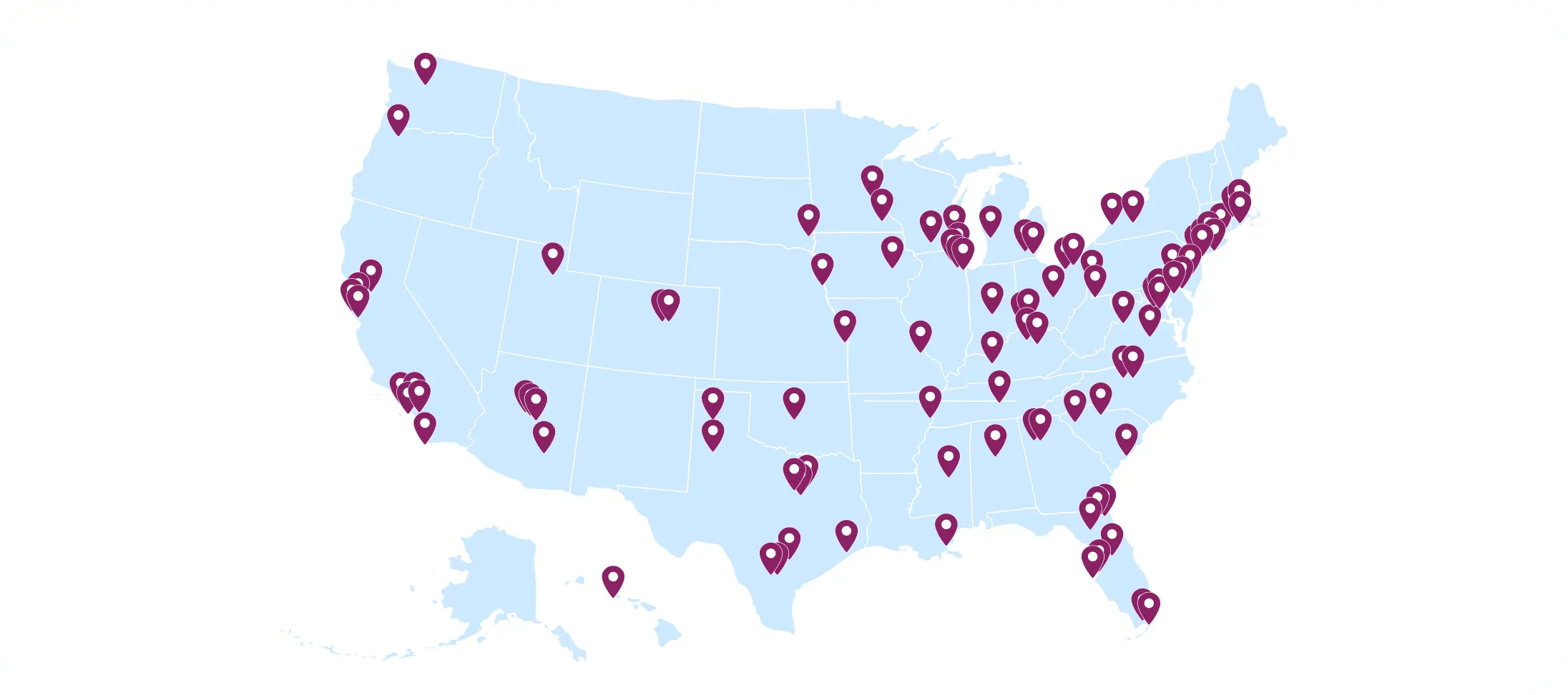
Active investigational transplant center sites in our clinical trials network
We harness established relationships with a contracted network of nearly 250 investigational transplant center sites as well as donor center and research lab partners across the United States.
Our established relationships and contracts with investigational sites allow us to successfully recruit sites to participate in trials, complete site start up faster and expedite processes throughout the duration of the study.
We’re ready to connect
Find out how our CIBMTR CRO Services professionals can support your cell and gene therapy trials.
