We provide clinical-grade leukopaks, both non-mobilized and mobilized, with customization options to fit development needs.

Leukopaks for Cell Therapy Manufacturing
We provide clinical-grade leukopaks, both non-mobilized and mobilized, with customization options to fit development needs.

Reliable, high-quality GMP leukopaks
Our standard GMP leukopak is designed to be delivered quickly without sacrificing quality. Optional add-ons to the standard leukopak—including cryopreservation—offer flexibility to meet developer needs. This approach allows for ease in scale up as therapies advance into later clinical trial and commercial phases.
NMDP BioTherapies standard GMP leukopak
NMDP BioTherapies developed our clinical-grade leukopak offering with input from clinical- and commercial-phase cell and gene therapy companies. This means we are prepared to meet a variety of supply needs for cell therapy manufacturing. A drug master file is on file with the FDA for our standard offering, to streamline regulatory filings for our partners.
In addition to the standard leukopak, we offer custom options to meet whatever needs our partners may have, including additional product testing and regulatory regions, and downstream cell processing (e.g., cell isolation).
All NMDP BioTherapies’ leukopaks include:

Fresh, clinical-grade leukopak

US. Food and Drug Administration (FDA) compliance

Standard donor management

Collections at qualified apheresis centers contracted to support standard leukopak collection

Standard collection and logistics processes and documentation

Drug master file (for the Standard Leukopak)
Clients can choose to add on:

Specified donor demographics

Additional donor samples for client-directed testing

Additional donor testing prior to collection

Standard product cryopreservation

Post-collection testing

Compliance for: Health Canada (HC), European Medicines Agency (EMA), and Therapeutic Goods Administration (TGA) compliance
Typical timeline to first product delivery
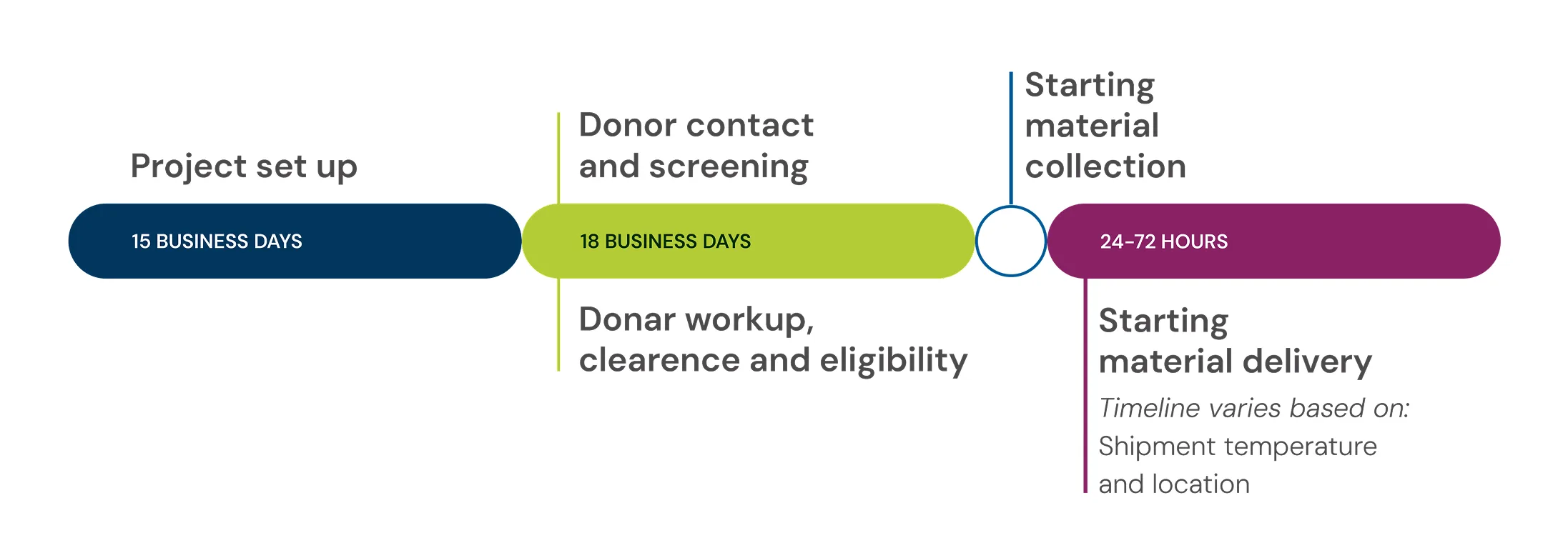
Note: Manufacturing slot or other sponsor-required scheduling/receipt criteria may impact first product delivery.
In most cases, the first product delivery for the standard fresh leukopak takes place in six weeks. However, the selection of add-ons is likely to increase time to first product delivery. The typical timeline, without add-ons, includes:
- Product set up in about 15 business days.
- Donor contact and screening plus donor workup, clearance and eligibility in approximately 18 business days.
- Starting material delivery of fresh products 24 to 72 hours after collection (timeline varies based on location and selected logistics).
Typical timeline to first product delivery
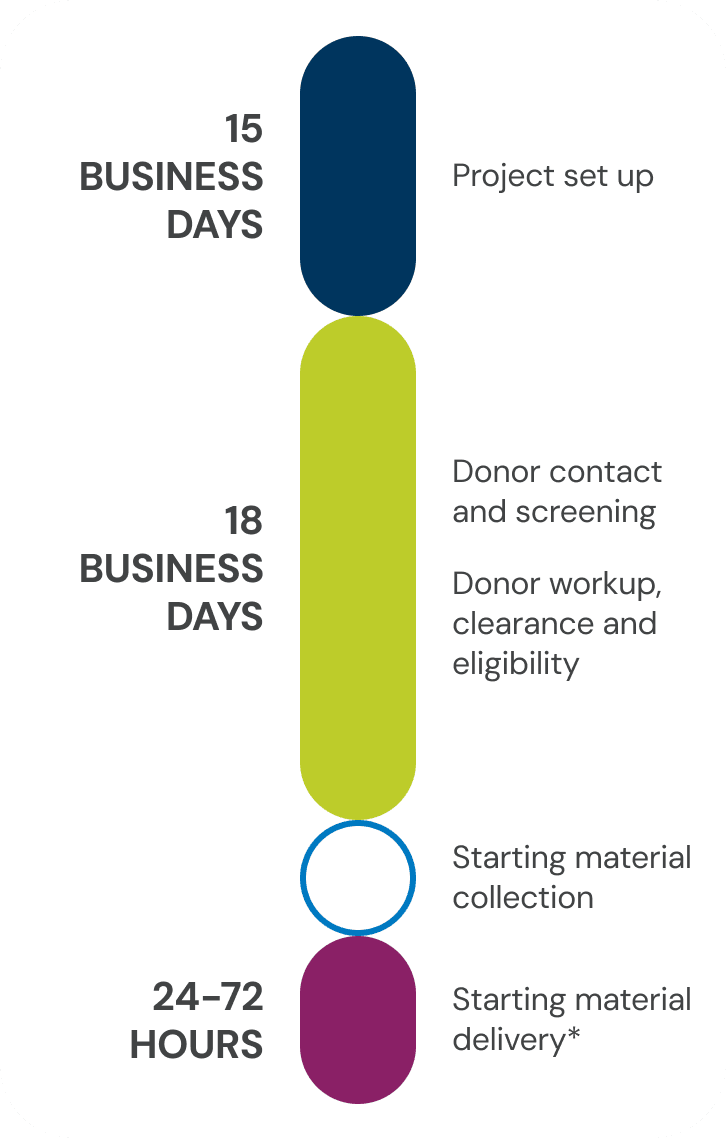
Note: Manufacturing slot or other sponsor-required scheduling/receipt criteria may impact first product delivery.
In most cases, the first product delivery for the standard fresh leukopak takes place in six weeks. However, the selection of add-ons is likely to increase time to first product delivery. The typical timeline, without add-ons, includes:
- Product set up in about 10 business days.
- Donor contact and screening plus donor workup, clearance and eligibility in approximately 18 business days.
- Starting material delivery of fresh products 24 to 72 hours after collection (timeline varies based on location and selected logistics).
Explore matched offerings
Find solutions for allogeneic cellular material that is specifically patient-matched