Our history of delivering time-critical cell therapies to patients means a future of advancing next-generation cell and gene therapies with companies like yours. Because together we can bring more cures to more patients.

NMDP BioTherapies History
Our history of delivering time-critical cell therapies to patients means a future of advancing next-generation cell and gene therapies with companies like yours. Because together we can bring more cures to more patients.

Successful cell therapy management and delivery for 35+ years
Our mission statement is clear: We save lives through cellular therapy. At NMDP BioTherapies℠, we believe every patient should have access to the best therapy to treat their disease.
We were established as part of NMDP℠, a pioneer in the field of one of the first cell therapies—hematopoietic stem cell transplant (HSCT). While we became an independently operating subsidiary in 2024 to focus on advancing cell and gene therapies, we still work closely with NMDP, continuing to leverage infrastructure and expertise.
NMDP and our partners have helped improve HSCT availability and outcomes for patients around the world.
We’ve done so through decades of experience, a global cell collection network and the CIBMTR® (Center for International Blood and Marrow Transplant Research®), a research collaboration with the Medical College of Wisconsin.
But our leaders knew a transplant isn’t always the best therapy for every patient. They also knew the experience, infrastructure and relationships we developed over decades could help cell and gene therapy companies bring emerging therapies to patients sooner.
From that knowledge, NMDP BioTherapies emerged.
Extending our life-saving mission to new cell and gene therapies
In 2016, we began partnering with leading cell and gene therapy companies to save more lives through autologous and allogeneic cellular therapy.
NMDP BioTherapies signed our first contract in February 2016 to provide cell sourcing and supply chain management services for the development of allogeneic cell therapies.
Less than two months later, we successfully delivered the first cellular starting material for manufacture to our partner.

Today, NMDP BioTherapies works with more than 60 cell and gene therapy companies. These companies range from small start-ups to large pharmaceutical companies. They rely on the expertise our team developed over more than 35 years of delivering time-critical cell therapies to patients.
Extending our life-saving mission to new cell and gene therapies
In 2016, we began partnering with leading cell and gene therapy companies to save more lives through autologous and allogeneic cellular therapy.
NMDP BioTherapies signed our first contract in February 2016 to provide cell sourcing and supply chain management services for the development of allogeneic cell therapies.
Less than two months later, we successfully delivered the first cellular starting material for manufacture to our partner.

Today, NMDP BioTherapies works with more than 60 cell and gene therapy companies. These companies range from small start-ups to large pharmaceutical companies. They rely on the expertise our team developed over more than 35 years of delivering time-critical cell therapies to patients.
A recent history with deep roots in cellular therapy
While NMDP BioTherapies has a recent history, our roots are deep as part of NMDP.
You may know NMDP for managing the NMDP Registry℠. It is the world’s most diverse registry of volunteer blood stem cell donors. But you might not know how managing the registry led to systems, processes and a network of contracted centers that make us the ideal partner for cell and gene therapy companies.
From the first cell therapy delivered to 135,000
The story starts in 1987 when NMDP managed our first donor search and bone marrow transplant. It was just one year after our establishment as the national registry of volunteer bone marrow donors for the United States.
Since then, our organization has managed more than 135,000 cell therapies, bringing life-saving therapies to people around the globe.
We did so by developing cell sourcing expertise and a predictive search algorithm, HapLogic℠, for customized donor selection. And by developing the relationships and expertise to navigate cell therapy supply chain complexities, coordinate logistics and deliver time-critical therapies on time.

From the first cell therapy delivered to 135,000
The story starts in 1987 when NMDP managed our first donor search and bone marrow transplant. It was just one year after our establishment as the national registry of volunteer bone marrow donors for the United States.
Since then, our organization has managed more than 135,000 cell therapies, bringing life-saving therapies to people around the globe.
We did so by developing cell sourcing expertise and a predictive search algorithm, HapLogicSM, for customized donor selection. And by developing the relationships and expertise to navigate cell therapy supply chain complexities, coordinate logistics and deliver time-critical therapies on time.

Investing in the future of cell therapy through research
During that time, we invested in cell therapy research. Our goal: to expand treatment options and find answers to save more lives and improve the quality of life for patients.
For example, in 2001, we built our Research Sample Repository. It’s one of the world’s largest tissue sample storage facilities used for medical research. The Repository provides researchers with access to more than 195,000 samples in inventory, with multiple aliquots available for each sample.

Investing in the future of cell therapy through research
During that time, we invested in cell therapy research . Our goal: to expand treatment options and find answers to save more lives and improve the quality of life for patients.
For example, in 2001, we built our Research Sample Repository. It’s one of the world’s largest tissue sample storage facilities used for medical research. The Repository provides researchers with access to more than 195,000 samples in inventory, with multiple aliquots available for each sample.

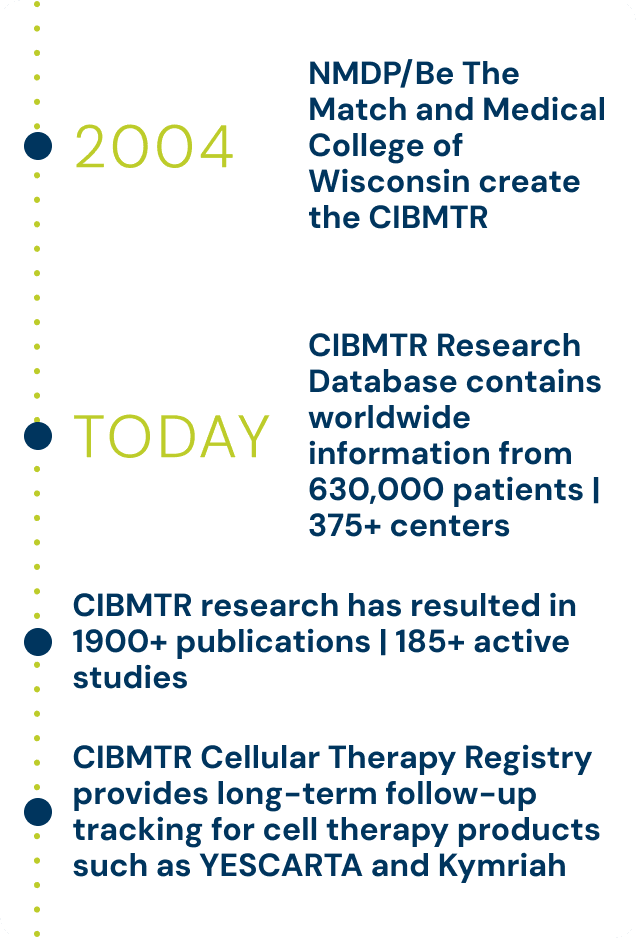
In 2004, NMDP and the Medical College of Wisconsin partnered to merge research operations and create the CIBMTR. The CIBMTR’s data spans 45 years of health outcomes data worldwide that was collected for decades prior to partnering.
Today, its research database contains information on more than 630,000 patients from more than 375 different centers.

The organization knows research is the only way to save more lives and enrich patients’ quality of life. Since inception, the collaboration has resulted in more than 1,900 publications. The CIBMTR currently has more than 185 active studies.
The CIBMTR implemented a Cellular Therapy Registry that accurately captures the nature, sequence and effects of modern cellular therapies, including CAR-T cells. Today the CIBMTR provides long-term follow-up tracking for the first two FDA-approved CAR-T therapies, Kite’s YESCARTA® and Novartis’s Kymriah®, among others.
In 2004, NMDP and the Medical College of Wisconsin partnered to merge research operations and create the CIBMTR. The CIBMTR’s data spans 45 years of health outcomes data worldwide that was collected for decades prior to partnering.
Today, its research database contains information on more than 630,000 patients from more than 375 different centers.
The organization knows research is the only way to save more lives and enrich patients’ quality of life. Since inception, the collaboration has resulted in more than 1,900 publications. The CIBMTR currently has more than 185 active studies.
The CIBMTR implemented a Cellular Therapy Registry that accurately captures the nature, sequence and effects of modern cellular therapies, including CAR-T cells. Today the CIBMTR provides long-term follow-up tracking for the first two FDA-approved CAR-T therapies, Kite’s YESCARTA® and Novartis’s Kymriah®, among others.
Partnerships support the development of next-generation cell and gene therapies
Today, our partnerships extend beyond the 50+ cell and gene therapy companies we work with to provide services for their allogeneic and autologous therapies.
We’ve developed strategic partnerships with many service providers whose focused expertise complements our own. Their expertise ranges from cell and gene therapy manufacturing to temperature-controlled logistics to sophisticated cell and gene therapy supply chain tracking.
This allows us to provide integrated solutions and improved operations for cell and gene therapy companies.
Partner with us
While we’re proud of our history, we’re looking forward to partnering with more cell and gene therapy companies on the development of next-generation therapies.








